Set TDT®
Description / Use
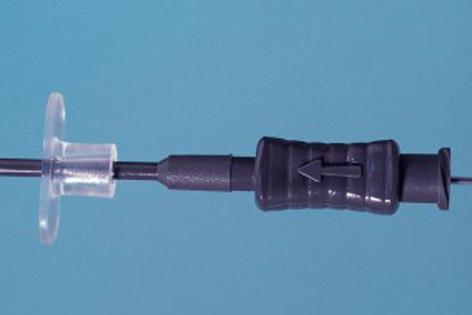
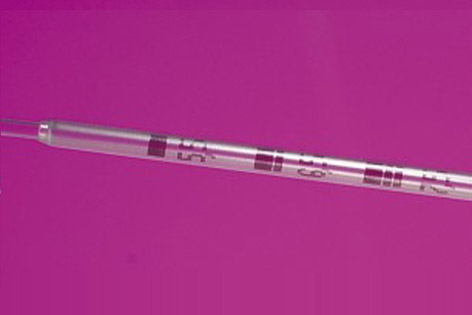
The Set TDT® includes:
- An outer sheath with a flexible extremity and 2 hysterometry guide-marks at 5.5 cm and 6.5 cm from the distal extremity.
- A malleable metal stylet coated in polyethylene.
- An extra-fine transfer catheter on a stainless steel micro tube of 0.50 mm in diameter in its proximal section for better handling.
In cervical stenosis, a thin malleable outer sheath and stylet can help overcome an obstacle before the loaded transfer catheter is gently and safely slid through the sheath.
| Reference | Description | Packaging |
|---|---|---|
| 1308000 | Set TDT® | Box of 10 units |